
An epimer is a stereoisomer that differs in configuration at a single stereocenter. Glycoside formation locks the anomeric carbon such that it can not undergo mutarotation. An anomer is one of a pair of stereoisomers (epimers) that have different configurations at the anomeric carbon. Condensation can occur with alcohols, amines, and amides. Glycosides: A general term referring to monosaccharide derivatives in which the functional group involving the anomeric carbon has an acetal or ketal structure. Β form: The configuration of a cyclic monosaccharide where the oxygen attached to the anomeric carbon is on the same side of the ring as the CH₂ OH group.\)) precipitate.ġ4. Α form: The configuration of a cyclic monosaccharide where the oxygen attached to the anomeric carbon is on the opposite side of the ring from the CH₂ OH group. A pair of stereoisomers that differ in configuration at the anomeric carbon are called This problem has been solved You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Stereoisomers or optical isomers: Molecules that differ three-dimensionally by the placement of substituents around one or more atoms in a molecule.Ĭhiral carbon (asymmetric carbon):A carbon that is attached to four different types of atoms or groups of atoms.Īnomeric carbon: A carbon derived from the carbonyl carbon (the ketone or aldehyde functional group) of the open-chain form of the carbohydrate molecule.
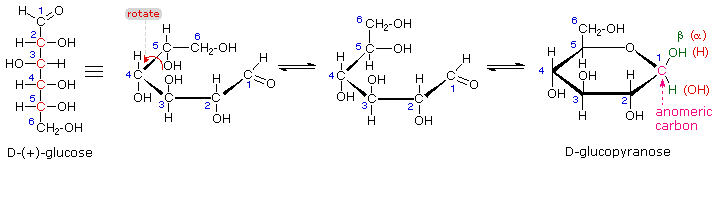
For example, α-D-glucose and β-D-glucose below are anomers. The carbon atom that forms the new chiral center (C-1) is called the anomeric carbon.Īnomers are special cases of epimers that differ in position at the anomeric carbon in particular. If the OH group is on the right hand side, the monosaccharide is of form. the configuration of the chiral carbon the carbonyl group determines whether a monosaccharide is D or L. When a molecule such as glucose converts to a cyclic form, it generates a new chiral center at C-1. a hormone that stimulates the liver to release glucose into the bloodstream when levels are low. In a Fischer projection, which chiral carbon determines whether the sugar is the D- or the L-isomer a. You can see D-allose, its just different at this one chiral carbon right here. They dont differ at every single carbon from glucose. The anomeric carbon has hemiacetal or hemiketal bonds with both of its. only carbon attached to two oxygens-OH may point up or down. Anomers differ in 3D arrangement at the anomeric carbon, which is a carbon atom found in the carbon chain of reducing sugars.
STERIOISOMERS THAT DIFFER AT ANOMERIC CARBON FREE
the end of a multi sugar chain with a free anomeric carbon. Now, all of these are stereoisomers but they differ at maybe just one. atoms are connected in the same order have different spatial arrangements.
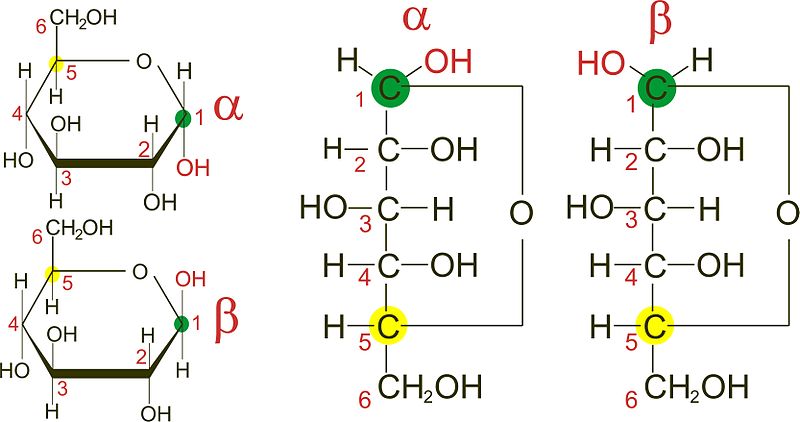
Glucose and mannose are epimers that differ at the C-2 carbon, while glucose and galactose are epimers that differ at the C-4 carbon, as shown below. You see that D-glucose and L-glucose are enantiomers, they differ at every single carbon. In the examples below, the difference in the position of the hydroxyl (OH) at one chiral carbon creates a pair of epimers. In carbohydrate chemistry, a pair of anomers is a pair of near-identical stereoisomers or diastereomers that differ at only the anomeric carbon, the carbon. Epimers and anomers are types of stereoisomers of carbohydrates that differ in the position at a single carbon atom.Įpimers are stereoisomers that differ in the configuration of atoms attached to a chiral carbon.


 0 kommentar(er)
0 kommentar(er)
